Oxygen Atom
Formal charge is determined by assuming that all bonding electrons are shared equally by the bonded atoms. If you draw a dot structure showing the sulfur atom single bonded to each of the four oxygen atoms, the formal charge on each oxygen atom is. Oxygen is a chemical element with atomic number 8 which means there are 8 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Bromite has one less oxygen atom than bromate. Dihydrogen hypophosphite ion is H 2 PO 2 −. Hypophosphite has two less oxygen atoms than phosphate, PO 4 3 −. The dihydrogen part of the name indicates that two H + ions have been added to hypophosphite, PO 2 3 −.
The key difference between atomic oxygen and molecular oxygen is that the atomic oxygen is highly reactive and does not exist in the atmosphere as it is whereas the molecular oxygen is less reactive and exists in the atmosphere as it is. Moreover, atomic oxygen is a free radical having the symbol O(3P) while the molecular oxygen is a diatomic oxygen having the symbol O2.
Oxygen is a chemical element having the atomic number 8. But when we refer to oxygen in common use, we are talking about molecular oxygen that we breathe. It has two oxygen atoms bonded to each other via covalent bond. Atomic oxygen has one oxygen atom. Therefore, it cannot exist as an individual chemical species because of its high reactivity.
CONTENTS
1. Overview and Key Difference
2. What is Atomic Oxygen
3. What is Molecular Oxygen
4. Side by Side Comparison – Atomic Oxygen vs Molecular Oxygen in Tabular Form
5. Summary
What is Atomic Oxygen?
Atomic oxygen is a very reactive chemical species having the symbol O(3P). It is a free radical. This means atomic oxygen has an unpaired electron that makes this atom highly reactive. Therefore, this atom does not exist naturally for even a short period; it tends to react with other chemical elements or compounds to become stable by pairing its unpaired electron.
However, in outer space, about 96% of oxygen exists as atomic oxygen because there UV radiation results in a low earth orbits atmosphere. This chemical species plays a major role in corrosion in space. Corrosion in space is the corrosion of materials occurring in outer space.
What is Molecular Oxygen?
Molecular oxygen is diatomic oxygen having the symbol O2. It contains two oxygen atoms bonded to each other via covalent bonding. There is a double bond between these two atoms. Since the two oxygen atoms have eight electrons around them, the oxygen molecule is less reactive.
Figure 02: Formation of Molecular Oxygen
Therefore, this chemical species exist in the atmosphere itself. Our atmosphere has about 21% molecular oxygen. This amount of oxygen is essential for all the organisms for their respiration. It exists as a colorless gas and the boiling point is −183 °C.
What is the Difference Between Atomic Oxygen and Molecular Oxygen?
Atomic oxygen is a very reactive chemical species having the symbol O(3P). It does not exist naturally for even a short period, but in outer space, it is the predominant form of oxygen. Moreover, it is highly reactive. Molecular oxygen is diatomic oxygen which has the symbol O2. It exists as itself in our atmosphere (about 21%). In addition, it is less reactive.
Summary – Atomic Oxygen vs Molecular Oxygen
Atomic and molecular oxygen are chemical species derived from the chemical element, oxygen which has the atomic number 8. The difference between atomic oxygen and molecular oxygen is that atomic oxygen is highly reactive and does not exist in the atmosphere as it is whereas molecular oxygen is less reactive and exists in the atmosphere as it is.
Reference:
1. “Allotropes of Oxygen.” Wikipedia, Wikimedia Foundation, 26 May 2018. Available here
2. Leenhouts, Doug. “The Differences of Oxygen & Oxygen Gas.” Sciencing, 24 Apr. 2017. Available here
Image Courtesy:
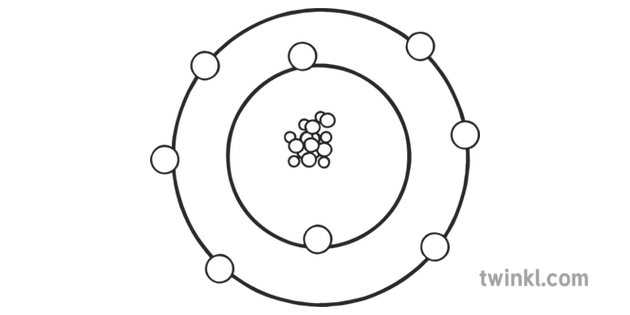
1.’Electron shell 008 Oxygen – no label’By Pumbaa (original work by Greg Robson) (CC BY-SA 2.0 uk) via Commons Wikimedia
2.’Figure 02 01 09’By CNX OpenStax,(CC BY 4.0) via Commons Wikimedia
Related posts:
The Element Oxygen
Oxygen ('Octium') is a chemical element in the periodic table that has the symbol O and atomic number 8. The element is very common, found not only on Earth but throughout the universe. Molecular oxygen (O2) (often called free oxygen) on Earth is thermodynamically unstable. Its initial appearance was due to the action of photosynthetic anaerobes and its ubiquity in later epochs has been largely facilitated by terrestrial plants, which release oxygen during photosynthesis.
| |||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | Oxygen, O, 8 | ||||||||||||||||||||||||
| Chemical series | nonmetals | ||||||||||||||||||||||||
| Group, Period, Block | 16 (VIA), 2 , p | ||||||||||||||||||||||||
| Density, Hardness | 1.429 kg/m3(273K), NA | ||||||||||||||||||||||||
| Appearance | colorless | ||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Atomic weight | 15.9994 amu | ||||||||||||||||||||||||
| Atomic radius (calc.) | 60 (48) pm | ||||||||||||||||||||||||
| Covalent radius | 73 pm | ||||||||||||||||||||||||
| van der Waals radius | 152 pm | ||||||||||||||||||||||||
| Electron configuration | [He]2s22p4 | ||||||||||||||||||||||||
| e- 's per energy level | 2, 6 | ||||||||||||||||||||||||
| Oxidation states (Oxide) | -2,-1 (neutral) | ||||||||||||||||||||||||
| Crystal structure | cubic | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| State of matter | gas (paramagnetic) | ||||||||||||||||||||||||
| Melting point | 50.35 K (-368.77 °F) | ||||||||||||||||||||||||
| Boiling point | 90.18 K (-297.08 °F) | ||||||||||||||||||||||||
| Molar volume | 17.36 ×10-6 m3/mol | ||||||||||||||||||||||||
| Heat of vaporization | 3.4099 kJ/mol | ||||||||||||||||||||||||
| Heat of fusion | 0.22259 kJ/mol | ||||||||||||||||||||||||
| Vapor pressure | __ Pa at __ K | ||||||||||||||||||||||||
| Speed of sound | 317.5 m/s at 293 K | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Electronegativity | 3.44 (Pauling scale) | ||||||||||||||||||||||||
| Specific heat capacity | 920 J/(kg*K) | ||||||||||||||||||||||||
| Electrical conductivity | ND 106/m ohm | ||||||||||||||||||||||||
| Thermal conductivity | 0.02674 W/(m*K) | ||||||||||||||||||||||||
| 1st ionization potential | 1313.9 kJ/mol | ||||||||||||||||||||||||
| 2nd ionization potential | 3388.3 kJ/mol | ||||||||||||||||||||||||
| 3rd ionization potential | 5300.5 kJ/mol | ||||||||||||||||||||||||
| 4th ionization potential | 7469.2 kJ/mol | ||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SI units & STP are used except where noted. | |||||||||||||||||||||||||
Notable characteristics
At standard temperature and pressure, oxygen is found as a gas consisting of two oxygen atoms, chemical formula O2. This oxygen is an important component of air, produced by plants during photosynthesis and is necessary for animals' respiration. The word oxygen derives from two words in Greek, the Greek oxus (acid) and geinomai (engender). (A misnomer, as there are many acids which do not contain oxygen.)

Liquid oxygen and solid oxygen have a light blue color and both are highly paramagnetic. Liquid oxygen is usually obtained by the fractional distillation of liquid air.
Applications
Oxygen finds considerable use as an oxidizer, with only fluorine having a higher electronegativity. Liquid oxygen finds use as an oxidizer in rocket propulsion. Oxygen is essential to respiration, so oxygen supplementation has found use in medicine. People who climb mountains or fly in airplanes sometimes have supplemental oxygen supplies (as air). Oxygen is used in welding, and in the making of steel and methanol.
Oxygen, as a mild euphoric, has a history of recreational use that extends into modern times. Oxygen bars can be seen at parties to this day. In the 19th century, oxygen was often mixed with nitrous oxide to promote a kind of analgesic effect.
History
Oxygen was discovered by the Swedish pharmacist Karl Wilhelm Scheele in 1771, but this discovery was not immediately recognized, and the independent discovery by Joseph Priestley on August 1st 1774 was more widely known. It was named by Antoine Laurent Lavoisier in 1774.
Occurrence
Oxygen is the most abundant element in the Earth's crust, estimated to comprise 46.7% of it. Oxygen comprises about 87% of the oceans (as H2O, water) and 20% of the atmosphere of Earth (as O2, molecular oxygen, or O3, ozone). Oxygen compounds, particularly metal oxides, silicates (SiO44-) and carbonates (CO32-), are commonly found in rocks and soil. Frozen water is a common solid on the outer planets and comets. The ice caps of Mars are made of frozen carbon dioxide. Oxygen compounds are found throughout the universeand the spectrum of oxygen is often seen in stars.
Compounds
Oxygen Atom Structure
Due to its electronegativity, oxygen forms chemical bonds with almost all other elements (which is the origin of the original definition of oxidation). The only elements to escape the possibility of oxidation are a few of the noble gases. The most famous of these oxides is of course hydrogen oxide, or water (H2O). Other well known examples include compounds of carbon and oxygen, such as carbon dioxide (CO2), alcohols (R-OH), aldehydes, (R-CHO), and carboxylic acids (R-COOH). Oxygenated radicals such as chlorates (ClO3-), perchlorates (ClO4-), chromates (CrO42-), dichromates (Cr2O72-), permanganates (MnO4-), and nitrates (NO3-)are strong oxidizing agents in and of themselves. Many metals such as Iron bond with oxygen atoms, iron (III) oxide (Fe2O3). Ozone (O3) is formed by electrostatic discharge in the presence of molecular oxygen. A double oxygen molecule (O2)2 is known, found as a minor component of liquid oxygen. Epoxides are ethers in which the oxygen atom is part of a ring of three atoms.
Isotopes
Oxygen Atomic Number
Oxygen has three stable isotopes and ten known radioactive isotopes. The radioisotopes all have half lives of less than three minutes.
Precautions
Oxygen can be toxic at elevated partial pressures.
Oxygen Atom Picture
Certain derivatives of oxygen, such as ozone (O3), hydrogen peroxide, hydroxyl radicals and superoxide, are also highly toxic. The body has developed mechanisms to protect against these toxic species. For instance, the naturally-occurring glutathione can act as an antioxidant, as can bilirubin which is normally a breakdown product of hemoglobin. Highly concentrated sources of oxygen promote rapid combustion and therefore are fire and explosion hazards in the presence of fuels. This is true as well of compounds of oxygen such as chlorates, perchlorates, dichromates, etc. Compounds with a high oxidative potential can often cause chemical burns.
The fire that killed the Apollo 1 crew on a test launchpad spread so rapidly because the pure oxygen atmosphere was at normal atmospheric pressure instead of the one third pressure that would be used during an actual launch. (see partial pressure)
See also
- Winkler test for dissolved oxygen for instructions on how to determine the amount of oxygen dissolved in fresh water.
- Combustion.
- Oxidation.
- The role of Oxygen as a diving breathing gas.
Reference
- Los Alamos National Laboratory – Oxygen(http://periodic.lanl.gov/elements/8.html)
External links
Oxygen Atomic N
- Los Alamos National Laboratory – Oxygen(http://periodic.lanl.gov/elements/8.html)
- WebElements.com – Oxygen(http://www.webelements.com/webelements/elements/text/O/index.html)
- EnvironmentalChemistry.com – Oxygen(http://environmentalchemistry.com/yogi/periodic/O.html)
- It's Elemental – Oxygen(http://education.jlab.org/itselemental/ele008.html)
- Oxygen Therapy – The First 150 Years(http://www.mtsinai.org/pulmonary/papers/ox-hist/ox-hist-intro.html)
- Oxygen Toxicity(http://members.tripod.com/tjaartdb0/html/oxygen_toxicity.html)
